Abstract
Current untargeted cytotoxic conditioning regimens for hematopoietic stem cell transplantation (HSCT) have been directly or indirectly associated with transplant related morbidity and mortality. Recent pre-clinical and early clinical data indicate that monoclonal antibody (mAb)-based immunotherapies targeting hematopoietic stem and progenitor cells (HSPC) are generally safe, opening the potential to significantly reduce the systemic toxicity related to conventional cytotoxic conditioning regimens. HSPCs express the receptor tyrosine kinase c-KIT (CD117) which is activated by its ligand stem cell factor (SCF) leading to c-Kit signaling that is crucial for HSPC biology. Blocking SCF binding prevents proliferation and leads to HSPC depletion in vivo. Therefore, CD117 is an attractive therapeutic target as a conditioning agent prior to HSCT. However, mAb-based conditioning necessitates a washout phase of the depleting antibody before engraftment of donor HSPC and prevents post-transplant redosing. HSPCs engineered to be shielded from a CD117-targeted immunotherapy could overcome this limitation and enable novel HSCT approaches.
Here, we present engineered CD117 variants that shield from a novel, concurrently developed, highly potent HSPC-depleting mAb (CIM058) while preserving CD117 function. We combined high resolution epitope mapping, computer-aided protein engineering and biophysical characterization of >40 variants bearing single amino acid substitutions to identify several CD117 variants that reduced CIM058 binding >1000-fold, in some cases resulting in no detectable binding even at high concentrations. All selected variants exhibit an SCF binding affinity in the range of wildtype (wt) CD117.
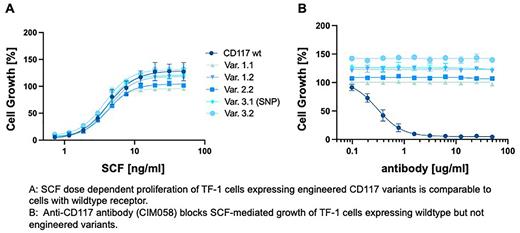
Variants were first introduced into the CD117 locus of the human SCF-dependent cell line TF-1 using CRISPR/Cas9-based homology directed repair (HDR). Biallelically edited TF-1 cells expressing CD117 variants retained SCF-dependent growth with EC50 values comparable to wt TF-1 cells (Fig. 1a). CIM058 dose-dependently inhibited cell growth of unedited but not engineered TF-1 cells (Fig. 1b). SCF induced phosphorylation of CD117 Tyr719 was dose-dependent in variant TF-1 even in the presence of the blocking antibody.
Similarly, select variants were engineered into mobilized peripheral blood HSPCs. CD117 variant expressing cells displayed hematopoietic differentiation potential in colony forming assays in vitro. Finally, CD117 variant 1.1 expressing HSPCs engrafted in immunodeficient NSG mice and contributed to multilineage hematopoiesis comparable to non-edited HSPCs. Intravenous administration of CIM058 effectively depleted CD117+ cells in mice receiving non-edited HSPCs. In contrast, in hosts receiving variant 1.1 engineered HSPCs, non-edited cells were depleted while edited CD117+ cells persisted.
In summary, we successfully identified and engineered functional CD117 variants harboring single amino acid substitutions. Cells expressing these CD117 variants were protected from binding by certain anti-CD117 mAbs, including the newly developed and potent antibody CIM058. Our data demonstrate the feasibility of selectively depleting wt HSPCs while sparing engineered, functional HSPCs, thus enabling future clinical development towards targeted and less toxic conditioning protocols for HSCT. Furthermore, CD117 shielded HSPCs could enable higher, longer and/or repetitive antibody dosing, allowing for toxin-free post-transplant adjustment of donor chimerism and targeted treatment of minimal residual disease in CD117+ malignancies.
Disclosures
Knezevic:Idorsia Pharmaceuticals: Ended employment in the past 24 months. Wellinger:Roche: Ended employment in the past 24 months. Winkler:Astra Zeneca: Ended employment in the past 24 months; Agios Pharmaceuticals: Ended employment in the past 24 months; Cimeio Therapeutics: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal